“You may encounter many defeats, but you must not be defeated. In fact, it may be necessary to encounter the defeats, so you can know who you are, what you can rise from, how you can still come out of it.” Maya Angelou
“Endure and persist; this pain will turn to good by and by.” Ovid
Introduction: For many of us, the dysfunction of our motor unit is our biggest rheostat for measuring the change or progression of Parkinson’s. It moves in small degrees, and slowly, over time, the change becomes apparent. The change in me is the occasional occurrence of frozen gait. This post will concern what I am trying to do to prevent it, and a subsequent blog post will be focused on the process of frozen gait.
“When obstacles arise, you change your direction to reach your goal; you do not change your decision to get there.” Zig Ziglar
New Symptom: My taking of immediate release (IR) carbidopa-levodopa has become like a rapid transition of on-to-off. While “on,” I can perform as usual, play golf, work out, go on walks, drive my car, sit at my desk, and type on my computer; essentially, I can do all that matters to me. By contrast, if I’m “off,” I tend to have somewhat of a frozen gait and difficulty walking and balancing. This change in me leads to the following five observations:
- I interpret this gradual transition as a progression of my Parkinson’s. I have been reading extensively about freezing gait and how to manage it. I have learned that the quality and quantity of sleep can sometimes influence the body’s response to the gait process. Good physical therapy is also available to train yourself to combat freezing gait.
- I can deal with the transition by being better aware of the timing between doses of my IR carbidopa-levodopa and by shortening the time between doses if necessary.
- Lack of sleep makes everything worse and magnifies the walking problem.
- The most significant transition is that when I wake up in the morning, if I have achieved more than 5 hours of sleep, my pot of dopamine is depleted, and I can barely walk until the first dose of levodopa kicks in. Thus, taking an extended form of carbidopa-levodopa could be ideal for this period.
- If this is successful, I suggest entirely switching to a long-term release carbidopa-levodopa form, which is ideal for the entire time, replacing IR carbidopa-levodopa. Unfortunately, the two best long-lasting products, Rytary and Crexont, are newer drugs without a generic product; thus, the cost of the medication may come into play.
“Nothing else is necessary but these – love, sincerity, and patience.” Swami Vivekananda
Brief Comparison of Controlled-Release (CR) Levodopa, and Extended Release (ER) Rytary and Crexont: Consolidating a bunch of literature reading, I find that the relative half-lives for these carbidopa-levodopa drugs are as follows:
| Product | Half-life (hr) |
| IR Carbidopa-Levodopa | 2 |
| CR Carbidopa-Levodopa | 4 |
| ER Carbidopa-Levodopa (Rytary) | 5 |
| ER Cabidopa-Levodopa (Crexont) | 6 |

“Our greatest weakness lies in giving up. The most certain way to succeed is always to try just one more time.” Thomas A. Edison
Controlled-release (CR) Carbidopa-Levodopa- I am trying to use this late at night. When I take it at ~11:00 PM (to replace my final IR carbidopa-levodopa dose), there is no difference when I wake up and have gotten ~5 hrs or more sleep. I wake up with some frozen gait until I get the first dose of IR carbidopa-levodopa in my system. However, I tried something last night; I took my final dose of IR carbidopa-levodopa at ~10 PM, fell asleep watching TV, woke up at 3 AM, took my dose of CR carbidopa-levodopa (50 mg-200 mg), and then went to bed. I woke up 5 hr later feeling ok, with fewer frozen steps than before. However, this is not an ideal continual scenario for me to reduce the frozen gait.
“It’s not a sin to get knocked down; it’s a sin to stay down” Carl Brashear
Visualizing the Effect of IR and CR Levodopa in Plasma as a Function of Time: I usedJohn Turner’s website, “Parkinson’s Measurement” (click here to access the site), to visualize and plot these results.
The starting data set is as follows. I take 13 tablets per day and apply one patch per day. The timing of the day is as follows, and it is based on a 4-hour timeframe between treatments:
7 AM, 3 tablets = 300 mg levodopa
11 AM, 2.5 tablets = 250 mg levodopa
3 PM, 2.5 tablets = 250 mg levodopa
7 PM, 2.5 tablets = 250 mg levodopa
11 PM, 2.5 tablets = 250 mg levodopa
Total amount of levodopa/day = 1300 mg.
7:15 AM, Neupro patch (6 mg, a levodopa-equivalence dose = 180 mg levodopa), which gives a levodopa equivalent daily dose (LEDD) = 1480 mg.
Study #2, substitute 1 CR 50 mg carbidopa-200 mg levodopa tablet (the bioavailability of CR levodopa is 70% compared to that found in IR levodopa) for the last dose of IR carbidopa-levodopa.
Study #3: Combine the two studies and take the CR levodopa tablet about 4 hours after the last IR levodopa tablet.
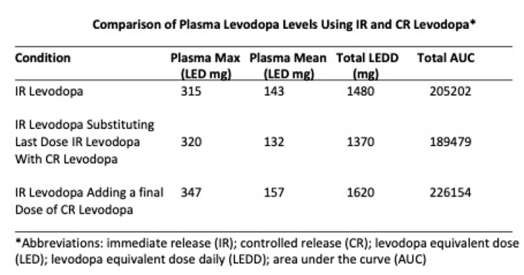
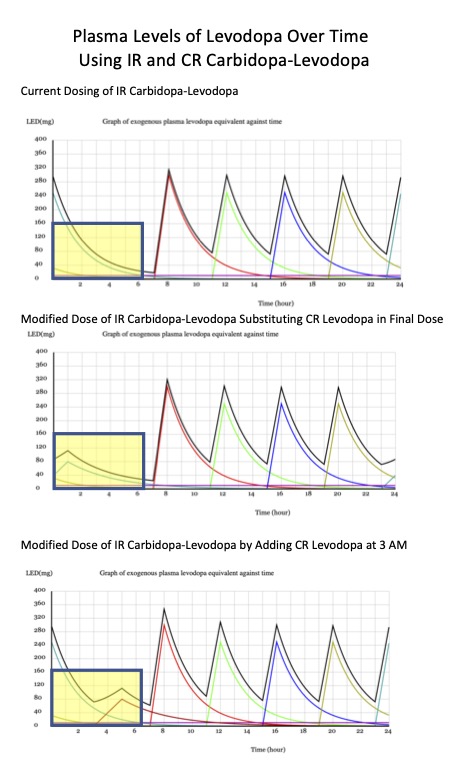
Please note the curves in the yellow boxes. They represent the sleep window between the last dose of levodopa and the first dose the following day. There appears to be a slight increase in individual levodopa when using the CR 50 mg carbidopa-200 mg levodopa capsule (middle and bottom panels) compared to the remaining levodopa in the top panel. However, the newer compounds described below will offer a wider therapeutic time frame between levodopa doses.
Koller, William C., J. Thomas Hutton, Eduardo Tolosa, Rudy Capilldeo, and Carbidopa/Levodopa Study Group. “Immediate-release and controlled-release carbidopa/levodopa in PD: a 5-year randomized multicenter study.” Neurology 53, no. 5 (1999): 1012-1012.
Livingston, Clare, and Laura Monroe-Duprey. “A review of levodopa formulations for the treatment of Parkinson’s disease available in the United States.” Journal of Pharmacy Practice 37, no. 2 (2024): 485-494.
“I have failed at times, but I have never stopped trying.” Rahul Dravid
Comparing Two Extended Release (ER) Carbidopa-Levodopa Preparations, Rytary, and Crexont:
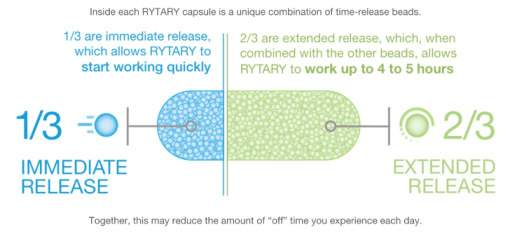
Rytary: This ER capsule of carbidopa-levodopa product has existed for several years, and Amneal Pharmaceuticals LLC makes it. The capsule is designed to release some IR carbidopa-levodopa and some CR carbidopa-levodopa. The idea is that if a person-with-Parkinson’s (PwP) is having difficulty with on-off periods, then the consistent release of carbidopa-levodopa should help level out this on-off transition. This schematic below helps explain how Rytary (originally named IPX066) works-
“To have striven, to have made the effort, to have been true to certain ideals – this alone is worth the struggle.” William Osler
Dosing Rytary to Replace IR Carbidopa-Levodopa: One of the obstacles in switching from the traditional IR carbidopa-levodopa product to an ER carbidopa-levodopa like Rytary is the actual dose to take each time and how many times per day. Ultimately, the goal of taking Rytary is to reduce the number of times one should take carbidopa-levodopa. The first part is that Rytary comes in different amounts of carbidopa-levodopa, and is illustrated below:

Clearly, from searching the literature on this topic, there was significant discussion from neurologists about prescribing and getting the correct dose of Rytary to their patients. I found the following papers to be most helpful and valuable in understanding the differences in how one should approach switching out IR carbidopa/levodopa with Rytary, including:
Optimizing the Dose of Rytary-
Hauser, Robert A. “How to dose carbidopa and levodopa extended-release capsules (Rytary).” Clin Med J 1, no. 2 (2015): 34-37.
Espay, Alberto J., Fernando L. Pagan, Benjamin L. Walter, John C. Morgan, Lawrence W. Elmer, Cheryl H. Waters, Pinky Agarwa,l et al. “Optimizing extended-release carbidopa/levodopa in Parkinson disease: consensus on conversion from standard therapy.” Neurology: Clinical Practice 7, no. 1 (2017): 86-93.
Silver, Dee E., and Richard M. Trosch. “Physicians’ experience with RYTARY (carbidopa and levodopa) extended-release capsules in patients who have Parkinson disease.” Neurology 86, no. 14_supplement_1 (2016): S25-S35.
Review of Rytary-
Hauser, Robert A., Leonid Zeitlin, Stanley Fisher, and Richard D’Souza. “Carbidopa and levodopa extended release capsules in patients with and without troublesome and non-troublesome dyskinesia.” Journal of Parkinson’s Disease 10, no. 3 (2020): 915-925.
Margolesky, Jason, and Carlos Singer. “Extended-release oral capsule of carbidopa–levodopa in Parkinson disease.” Therapeutic advances in neurological disorders 11 (2018): 1756285617737728.
“Men fail much oftener from want of perseverance than from want of talent.” William Cobbett
Rytary Dosing: Rytary dosing differs from IR carbidopa-levodopa (Sinemet). You must understand some differences if you are talking with your neurologist about trying it. Rytary comes in four different amounts and is in the standard carbidopa-levodopa one:four ratio is used but not amount-wise (see schematic above). Table 1 in the paper by Hauser (2015) is from the FDA-approved protocol for Rytary, reproduced here:
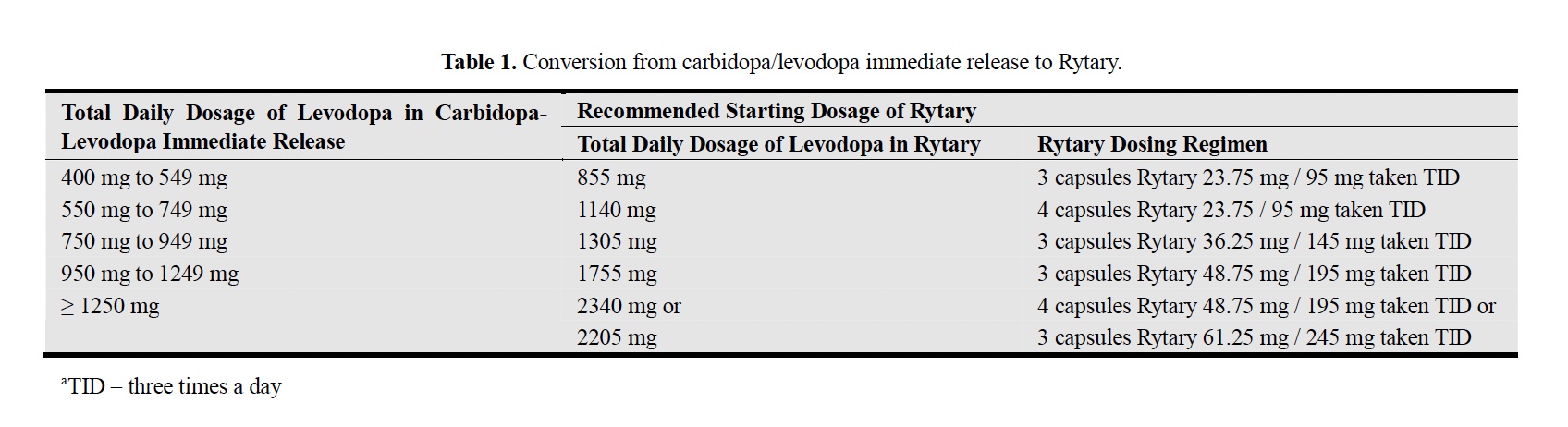
From this Table, one takes the total amount of IR levodopa equivalent being used by the PwP, and the initial thought is to take Rytary three times per day at the following dose. For example, a PwP is taking a total of 500 mg of IR carbidopa-levodopa. From this Table, it is suggested that the PwP take three capsules of the Rytary 23.75 mg carbidopa/95 mg levodopa and take it three times daily. For me taking 1300 mg IR carbidopa-levodopa in a day, it is suggested that I take either four capsules of Rytary 48.75 mg carbidopa/195 mg levodopa three times per day or three capsules of Rytary 61.25 carbidopa/245 mg levodopa three times per day. So, using this strategy for my one late-night dose of levodopa, I would use either one of these doses of Rytary, and I would also request to have 30 per month of the 23.75 mg carbidopa/95 mg levodopa to balance out the effect of changing from IR carbidopa-levodopa to Rytary.
Based on what my medical insurance would pay for the cost of Rytary, they would charge me USD 64 for <300 capsules of the Rytary 61.25 carbidopa/245 mg levodopa without further approval from my Neurologist. I would need 270 Rytary 61.25 carbidopa/245 mg levodopa capsules for a month, with some leeway to further optimize the evening dose with 30 of the 23.75 mg carbidopa/95 mg levodopa capsule of Rytary.
The most straightforward formula for switching from IR carbidopa-levodopa to Rytary utilizes a 2:1 conversion ratio (total daily Rytary dose of levodopa to current total daily IR levodopa dose). Another example, a PwP takes 600 mg IR carbidopa-levodopa 4 times per day and would probably start with two 36.25 mg carbidopa/145 mg levodopa capsules of Rytary 4 times per day (for a total of 1,160 mg levodopa, which gives a 1.93 ratio of Rytary levodopa to IR levodopa). The PwP could add, if needed, the 23.75 mg carbidopa/95 mg levodopa capsule of Rytary if symptom improvement is still required.
The three papers mentioned under “Optimizing the Dose of Rytary” describe how best to prescribe Rytary and the challenges faced by some PwP trying to convert from IR carbidopa-levodopa to Rytary.
One final note about Rytary is that a generic form of Rytary approved should become available in the USA around the end of July, 2025.
“Anytime we step out boldly to make changes, we take a chance that we might fail. But the only way to get better is to try.” Joyce Meyer
Crexont, Also Known as IPX203: The FDA recently approved another ER carbidopa-levodopa capsule, previously known as IPX203 and now known as Crexont. Amneal Pharmaceuticals, LLC, makes Crexont. Like Rytary, Crexont comes in four capsules containing IR and CR levodopa at different levels. Crexont appears to have the most extended half-life compared to other levodopa derivatives (see the Table that follows the Introduction).
The composition of the Crexont capsule is quite interesting. Rapid dissolution granules contain levodopa and carbidopa for rapid dissemination. There are extended-release beads with three properties: the beads are coated with a sustained-release polymer to provide a slow release of the drug, a mucoadhesive polymer to maintain the bead in the absorption area longer, and an enteric polymer to keep the bead from being quickly destroyed in the stomach. Crexont was created to improve the short plasma half-time of levodopa and the short absorption window in the gut for levodopa.
Crexont behaves similarly to Rytary, as it has an enhanced half-life of levodopa, making it an ideal candidate drug for anyone struggling to manage off-on cycles with their carbidopa-levodopa.
“Paralyze resistance with persistence.” Woody Hayes
Dosing of Crexont: There is only limited information regarding the dosing of Crexont as a replacement for IR carbidopa-levodopa. Dr. Hauser’s work clearly outlines the dosing of levodopa-containing medicines. To convert from IR carbidopa-levodopa to Crexont, multiply the most frequently used individual dose of IR carbidopa-levodopa by 2.8 and administer it an average of 2-4 times daily, using the patient’s judgment to titrate the adjustment. As with Rytary, this is the initial amount to reach the correct dose. For me, this translates to 250 mg of IR levodopa in IR carbidopa-levodopa multiplied by 2.8, resulting in 700 mg of levodopa. Thus, I should take two of the 87.5 mg carbidopa-350 mg levodopa capsules of Crexont, taken 3 times per day. Utilize the 35 mg carbidopa-130 mg levodopa capsule to titrate to the optimal amount of Crexont.
From the Crexont website, here is how they calculate the amount of Crexont I should take:

Using a single-dose study in patients with advanced Parkinson’s, Modi and colleagues compared Crexont to Rytary. This study found that Crexont achieved a higher plasma levodopa concentration, providing a longer clinical benefit. This research is described in the following paper: Modi, Nishit B., Aravind Mittur, Robert Rubens, Sarita Khanna, and Suneel Gupta. “Single-dose pharmacokinetics and pharmacodynamics of IPX203 in patients with advanced Parkinson’s: a comparison with immediate-release carbidopa-levodopa and extended-release carbidopa-levodopa capsules.” Clinical Neuropharmacology 42, no. 1 (2019): 4-8.
In general, Crexont appears to be an ideal drug for treating Parkinson’s, especially for those experiencing on-off issues. Clearly, Crexont has a superior plasma half-life compared to IR and CR carbidopa-levodopa and shows a slight advantage over Rytary. The novelty of Crexont seems to be a partially limiting factor, as healthcare experts are only just beginning to learn about its properties, and determining the correct dose poses challenges similar to those faced by Rytary upon its introduction. As Rytary prepares to become available as a generic product, and depending on insurance status and costs, individuals must carefully weigh the options between Rytary and Crexont.
Hauser, Robert A., Alberto J. Espay, Aaron L. Ellenbogen, Hubert H. Fernandez, Stuart H. Isaacson, Peter A. LeWitt, William G. Ondo et al. “IPX203 vs immediate-release carbidopa-levodopa for the treatment of motor fluctuations in Parkinson disease: the RISE-PD randomized clinical trial.” JAMA neurology 80, no. 10 (2023): 1062-1069.
LeWitt, Peter, Aaron Ellenbogen, Daniel Burdick, Steven Gunzler, Ramon Gil, Rohit Dhall, Ghazal Banisadr, and Richard D’Souza. “Improving levodopa delivery: IPX203, a novel extended-release carbidopa-levodopa formulation.” Clinical Parkinsonism & Related Disorders 8 (2023): 100197.
Hauser, Robert, Henry Moore, Ghazal Banisadr, and Richard D’Souza. “Optimizing a Patient’s Oral CD-LD Dosing Regimen Is Critical for Improving Efficacy in Parkinson’s Disease (P4-3.019).” In Neurology, vol. 102, no. 17_supplement_1, p. 3447. Hagerstown, MD: Lippincott Williams & Wilkins, 2024.
Hauser, R. A., H. H. Fernandez, J. Jimenez-Shahed, S. Allard, G. Banisadr, S. Fisher, and R. D’Souza. “Duration of “Good On” time per dose: Immediate-release carbidopa-levodopa vs. extended-release carbidopa-levodopa (IPX203, CREXONT®).” Parkinsonism & Related Disorders 131 (2025): 107239.
Marsili, Luca, Matteo Bologna, Lily Y. Chen, and Alberto J. Espay. “Treatment of Motor Symptoms of Parkinson’s Disease.” Neurologic Clinics (2025).
Conclusions: Our lives continue in the presence of Parkinson’s. The latest exhibition of a new symptom now includes frozen gait. The current state of my Parkinson’s argues that I remain aware of the timing of my on-off balance/imbalance. By doing so, I can stay covered adequately with levodopa to prevent this frozen gait. However, this might prove to be a more difficult transition with time.
“Life is not easy for any of us. But what of that? We must have perseverance and above all confidence in ourselves. We must believe that we are gifted for something, and that this thing, at whatever cost, must be attained.” Marie Curie
Cover Photo Image by Steffen Wachsmuth from Pixabay